 |
|
RECOMMENDED CONCISE
|
|
Side Effects, Effectiveness, Corporate Medicine
|
 |
 |
|
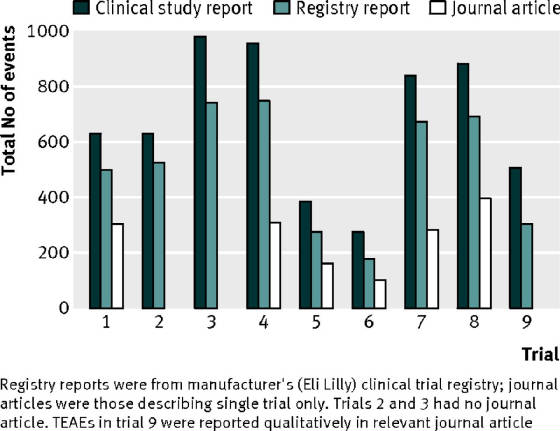
| Cymbalta, white bar is the journal reported side |
|
| effects, dark raw bar is the data collected |
This graph is the tip of the
iceberg. First pharma does not actively
solicit in a phase iii clinical trail all the side effects, or encourage
reporting of them via a patient questionnaire. Often serious side effects and minor ones are grouped
together given a less serious heading.
Finally the trials are too short to get figures on deaths, cancer, heart
attacks, and which such categories are missing in longer trials. Another bias is that those who drop out (cease
taking Cymbalta) are not sought after to find out why they dropped out of the
study. Trials are ran for marketing
objectives. The results of the trial
for
the blockbuster Cymbalta is owned by Eli Lily who resists all attempts to make the raw data
public.
Side Effects,
Effectiveness,
Corporate Medicine http://healthfully.org/rc/id9.html (7/13/13-jk)
1) A
fair assessment of side effects and benefits of a drug treatment requires
accurate, balanced, essential, and easily accessible information. On the one
side we have many conscientious
doctors; on the other side those who are PhARMA1 “friendly”. PhARMA has gained nearly total control of the
production of information about their products:
the research, the journals, the textbooks the media, the FDA, administrators,
treatment protocols, and the doctors’ continuing-education classes. Their
control of the production of information functions to maximize profits. For
general, concise summation issue-by-issue
account of what is wrong with corporate dominated drug industry read Marketing Science—with links to
journal articles. That article confirms
Harvard Prof. Dr. Marcia Angell's
assessment:
“We certainly
are in a health care crisis; ... If we had set
out to design the worst system that we could imagine, we couldn't have imagined
one as bad as we have.” What
follows supports Dr. Angell’s assessment: an account of the labyrinth of deceit concerning side effects and effectiveness of
drugs woven by Big PhARMA. Her book, The
Truth About Drug Companies, confirms the same. For people to make prudent
drug choices they
need to know the state of health care; an informed skepticism is
essential. Several documentaries
confirm this assessment;
two excellent ones are Money Matters
and Big Bucks, Big Pharma. A healthful
skepticism along with healthful advice is a starting
point for informed medical choices.
2) In the study of 22 elite athletes with familial
hypercholesterolemia (over twice the normal LDL level), 73% did not tolerate
the treatment; viz. they wouldn’t continue to take a
statin, though there condition is life-shortening. They had a mutation in FH, LDLR
or the ApoB which regulates the production of cholesterol. The article
describing their treatment found
that: “Regardless
of the biochemical background
statin therapy and top athletics seem to be almost incompatible.” Two studies
of insured elder found long-term compliance of 25 & 20%. Yet
medical textbooks write of statins “Safe, effective, and well-tolerated … that
treat disorders of lipid metabolism” (Braunwald, Heart Diseases, 8th
Ed, p 2286). This is the message taught
in textbooks, at the continuing education classes for physicians, and it is supported
by “marketing science” in the journal articles.
Unfortunately, the safe, effective, once-leading
treatment, a mega dose of Niacin
(vitamin B3) is not recommended, and when requested, the doctor’s advice is fundamentally wrong in ways discourages
its usage. Thus niacin has only 3% of
the market for treatment of hypercholesterolemia. Statins were original approved
by the FDA for
ONLY familial hypercholesterolemia (for good reasons). Once approved, the door was opened wide. Marketing
works, thus the deceptions of
well-tolerated has become accepted as the truth.
3) Not
Tolerated is a very low standard for side effects: it counts only those who stopped taking the drug because of side-effects—often
without listing the causes of dropping out. An even lower standard is hospitalization for side effects.
Even lower is a study
of Warfarin that counted as a
stomach bleed only those who required a transfusion of 2 or more pints of blood—the
same standard was used for Plavix (the second most profitable drug). The Plavix
& Warfarin sales reps will use
this result, without mentioning the transfusions, to inform the physician that their
drug is safer than aspirin.
4)
Going up the
ladder from not tolerated, the next higher standard is self-reporting, either
when asked or volunteered. Even higher would be self reporting based on
having the subject periodically fill-out
questionnaire that lists all likely side-effects. The highest standard--in an ideal world--would be an active
study of the general population of patients likely to use the drug in a
clinical setting, with special subset of the elderly & frail. This study
would include physical and mental
tests (such as treadmill and memory) and analysis for of our body’s biochemistry
pathways or metabolism would likely be affected by the medication, e.g. statins
block the mevalonate pathway thus affect CoQ10. FDA approval ought to be contingent upon a thorough,
independent evaluation. People ought
to come first. But given that the
market
place is against side effects
and we have a corporatist
state, the standards of scrutiny
are a façade, and deception is the norm.
What follow in this expose are four illustrative examples of PhARMA’s
marketing ploys.
5)
When the 800 pound
gorilla
wants to hide the extent of side effects, they do. Roche in 2011 failed to assess
80,000 reports
from physicians on side effects including 15,161,
deaths and to forward them to the European Medical Agency EMA), as required
by law. In June of 2012 the EMA began an
investigation. A fine of 5% of sales is legislated in
Europe. The FDA has a similar law
requiring the reporting of adverse events, but fines are not legislated. (Have
you heard of this scandal in our
corporate media?). This is the first
case in over 5 years investigated by the EMA.
Given the PhARMA friendly nature of the FDA and EMA, undoubtedly most
doctors don’t go through the trouble of voluntarily
filing reports. Physicians in Europe and
the
US are not required to report
side effects to the regulatory agency or Drug Company. Nevertheless given the voluntary
system, “more than 525,000 unique patient reports
were
submitted to AERS [FDA’s report monitoring system] in 2010, with 9.6% of those
adverse events resulting in patient deaths and nearly 21% resulting in patient
hospitalization…. Drug safety professionals
estimate that only 10% of adverse events are reported to the FDA every year,
thus grossly underestimating the number of actual adverse events associated
with a medication (Heinrich, 2000).” Many
side effects are not listed, others considered rare (though they aren’t), and
with multiple drugs being taken, even a conscientious physician will grossly
under-report side effects. Other side
effects are considered part of the condition, as the example of statins
increasing the risk of
death from a heart attack, especially among the elder—a fact denied by a company
sponsored studies and their thought
leaders. Quite a tip of the iceberg!
6) The problem
goes further than
PhARMA sitting on side-effect information sent to them from doctors. Everything is measured by its effects upon
marketing. For example, Phase III studies
(funded by PhARMA who
writes up the results) generates raw data
that will be submitted to the FDA--and like foreign regulatory agency—to
demonstrate that their drug is better than a placebo (nothing at all). There
is no requirement that they submit all
of the early research including those for phase I & II. Phase III studies are done for the sake of
gaining patented exclusivity from the FDA.
The FDA receives the raw data and decides if the drug as tested is
slightly better than nothing at all, a placebo.
Side effects at this stage of review are rarely factored into the
evaluation. Once the drug is approved
for a particular use, the study usually is published for the sake of marketing
the new drug (phase IV studies are
done after approval). Unfortunately the FDA does not review the journal articles that are based upon the raw data
submitted to the FDA in the
phase III studies. Moreover, the
journals never receive the raw data (unlike submission
in the other sciences), thus the journal’s review of the article cannot effectively
uncover bias. Knowing that there is no effective review,
the marketing departments of drug manufacturers freely manipulate the results
to promote the just approved drug. An
independent review of 37 phase III studies based upon the raw data (which was
obtained from the FDA under the freedom of
information act) compared the raw data
to the journal articles. The five
scientists who did this investigation of raw
data found for phase III Studies
there was an average
bias of 32%--range 11% to 69% (in NEJM Jan 2008). There
are many ways to get the paid for
results. For example, in selection 9) Merck
eliminated 1/3rd of those given Lipitor, because of reaction which
revealed through testing would have produced a negative outcome. Remember, bias
and the façade of review when
reading medical journals, and when hearing claims from the media and physicians
that a drug is safe and effective.
Consider the limitations placed by the withholding of the raw data, when
making a treatment decision.
7)
The further we
look into research and publication of medical science, the corporate difference
is observed. The vast majority of
clinical studies published in journal are phase
IV studies, which are designed by marketing departments solely for the promotion
of sales. Nearly all of these studies are well below the standards in phase III
studies. Some phase IV studies are
submitted to the FDA for extending patent life (see Harvard Prof. Marcia
Angell’s book The Truth about Drug
Companies and How they Deceive Us for
development of this complex topic). Some
are done to show that their drug is safer than an alternative, often
patent—another area of great abuse. Most are done to support new, off-label
uses
(not approved by FDA), which the sales reps and thought leaders7 of the pharmaceutical companies have a their
drug is safer than an alternative, often patent—another area of great abuse. Most are done to support new, off-label
uses
(not approved by FDA), which the sales reps and thought leaders7 of the pharmaceutical companies have a
right to use to promote their drug, a thing that their employer can’t actively
do (another regulation with a gaping hole).
In most cases off-label uses of a drug exceed the sales for the FDA
approved uses. Many of the phase-IV
studies are not studies at all, but merely a way to reward loyal doctors by
paying him for partaking in the study. He
then fills out a questionnaire about the drug’s effectiveness. It is also
a way to get a doctor to prescribe
their drug. Often the study is published
in an obscure journal with low standards.
These pseudo-science findings are then used to promote the drug.
8) There
are many, many ways of down-playing side effects. The most effective way
is to sell the drug’s
perceived benefits. The perceived
benefit of lowering coronary events has made statins the most used family of
drugs. By 2007 the world-wide sales of
Lipitor was $131 billion. Fully 50% of
men and 17% of women in the age group 65-74 have taken a statin drug in the
prior 30 day during the period 2005-2008, and 43% of men and 36% of women 75
and above. A small chorus warns that Statins are not worth taking. They are ignored by the
press, and buried
under a mountain of PhARMA financed studies. The large, seemingly-scientific TNT
(Treating to New Target) Study (2007), in 2007, in
2006, in
2006, also earlier
in 2005, and other articles, it produced ammo for thought leaders and sales reps
pushing Lipitor, who educate doctors
on newly discovered benefits of taking higher doses of Lipitor and treating
lower levels of cholesterol (expanding the category) and the American Heart
Association obliged PhARMA.
9)
Pfizer didn’t bury
all the details of the TNT Study. The British
Medical Journal (BMJ) article questioned the conclusion of the TNT study that “nearly
everyone ought to be on a statins”. The
4 authors explained Selection
bias for inclusion
in the study (2006): “The
low frequency of side effects in the TNT trial compared with the
IDEAL trial may be explained by the way patients were selected for
treatment. In the TNT trial more than 3000 people were excluded because
they did not fulfill the criteria, already had
raised aminotransferase concentrations, cancer, or another disease
associated with a limited lifespan, or for "other reasons. After
one-to-eight weeks' treatment with low dose atorvastatin [Lipitor], an additional 5429 patients were rejected,
including 197 with non-fatal clinical endpoints, 193 with adverse
events, 69 who did not comply with the treatment, 195 who had
ischaemic events, 15 with fatal clinical endpoints, and 373 for
other reasons. No information was provided on the nature of the reason
for excluding 3,000 for “other reasons”. Similarly, it is not clear which side
effects later caused 7.2% to stop the treatment.3 Finally, of the 18,468 patients
originally screened for the TNT trial, only 10 003 (54%) were
selected, whereas for the IDEAL trial the number was 91.7%, meaning
that the patients studied in the TNT trial were much healthier than
those included in the IDEAL trial and also than those seen in the
doctor's office.” Medical Journals
cannot challenge/offend their advertisers
who are also the source of articles, and their editors know this. Excluding
people from a study for to obtain
results is well below the standards of science, but not medicine. The
prestigious Lancet
published (among others) wrote a favorable review of the TNT study. Given this violation of ethics, what
confidence can be placed in Merck’s Zocor 1994, very profitable Scandinavian Survival
Study? Medical journal articles must be viewed as marketing tools, with no way
of confidently discerning what is accurate. Corporations rarely in the
60’s controlled
clinical trials, they funded medical schools to do them.
10) Metastudies put together the “best” published
articles and arrive at the averages. But
most are of these done by PhARMA friendly doctors. Protocol issues in these
metastudies are
buried because they group different doses, different members in a family of
drugs (such as progesterone and MPA for HRT),
and the universal bias of the journal articles results remains—average positive bias of 32% (see #6 above). When the critical, prestigious, non-profit,
organization, the Cochrane
Collaboration, which specializes in metastudies,
though not PhARMA friendly, unfortunately fails to adequately address these
issues. Cochrane and Worst Pill, though
failing to challenge the web of corruption, provide valuable guidance with a
critical slant to physicians and the public.
They will often when the evidence is clear challenge treatment protocols
when supported by convincing evidence.
11) Cochrane, for example, found gross
inconsistencies and the very suspect fact that 80% of studies by Roche’s
Tamiflu were unpublished. But these
missing studies can not fix the
inadequacies since PhARMA owns the raw data and does not share it. Cochrane requested missing studies in 2009
from Roche on Tamiflu, a treatment of influenza A and B viruses. They were told
it would be supplied. In 2012 they reversed their position on
Tamiflu after not receiving the data from Roche. This is understandable given
that the evidence
is thin for Tamiflu’s effectiveness.
Hardly enough to justify the billions of dollars that have been spent taking
that drug, and billions more by governments stockpiling the drug following the
avian-flu scare. Wikipedia: “The
efficacy of Oseltamivir [Tamiflu] is disputed, as a significant amount of its
clinical trial data remains unpublished by the drug's manufacturers. A
meta-analysis done by Kaiser et al. and supported by manufacturer Hoffmann-La Roche,
was published in 2003.[13] It
concluded that oseltamivir can prevent complications of influenza such as
pneumonia if it is taken within 48 hours
of the first appearance of influenza symptoms. Kaiser's study was based on a
summary of ten randomized controlled trials, of which only two had been published.
The unpublished nature of most of the included
trials would later cast doubts on Kaiser's conclusions.” In addition,
“As of
December 15, 2010, the World Health Organization (WHO) reported all 314 samples of the prevalent
2009
pandemic H1N1 flu tested worldwide have shown resistance to oseltamivir.[5]….
In the 2008-2009 flu season, the proportion of resistant H1N1 increased to
99.4%.... The Cochrane review reported a meta-analysis of 20 studies which
showed oseltamivir offered mild benefits
in terms of duration of symptoms for healthy adults if taken within 24 hours
of onset of symptoms
[few see a doctor that quickly], but found no clear evidence it prevented lower
respiratory tract infections or other complications of influenza.[14]
These findings relate only to its use in healthy adults with influenza, not in
patients judged to be at high risk of complications…. A subsequent Cochrane
review, in 2012, maintains that significant parts of the clinical trials still
remains unavailable for public scrutiny, and that the available evidence is not
sufficient to conclude that oseltamivir decreases hospitalizations from
influenza-like illnesses.[4]
They reversed their finding of an early
meta-analysis which found Tamiflu effective.
The later review found not only evidence lacking but also serious discrepancies. To resolve this Cochrane asked Roche to
supply the raw data in 2009, and Roche
agreed. Then after 2 years, the
request was made again. Roche now in
their response attached the condition that Cochrane must agree not to make
public the information. Cochrane
declined. “As of October 2012, 60% of
Roche's clinical data concerning oseltamivir remains unpublished.[17]”, Cochrane’s
position: “We
believe that until more is known about the mode of action of neuraminidase
inhibitors, health professionals, patients and other decision makers need to
reflect on the findings of this review before making any decision about the use
of the drug.” Cochrane certainly is not
the last word in critical analysis. They
missed the WHO lab studies which showed that the drug was 99% ineffective
against prevalent flu strains (see above).
12) Cochrane had also missed
the issues of side effects and journal bias.
No mention was made of side effects by Cochrane, though one had caused
quite a stir. “As of February
4, 2006, 39 deaths had been associated with
oseltamivir in Japan, 13 of which were of children aged 16 and under.5 Several
of the deaths in children involved falls from high places.6 In
2008 the FDA and Roche issued an advisory warning regarding neuropsychiatric
events associated with the use of oseltamivir. Most of these reports were from
Japan and
included delirium and abnormal behavior leading to injury, and in some cases
resulting in death”.9 WorstPill.org. More on this in
Wikipedia: “Roche then did its own study of 2,800
children using a professor of pediatrics at Yokohama University who found no
difference in behavior. But a carefully
constructed study by the Japanese Health Ministry found that children who took
oseltamivir were 54 percent more likely to exhibit abnormal behaviour than those
who did not take the drug”. What about
affecting adults? The journal articles
claim it to affect only children (good damage control by Roche, using their stage
iv studies). What of other side
effects? A http://scholar.google.com/ found
none. This is surprising given its bio
activity as a neuramidase inhibitor. This
rare serious side effect gives the appearance of effective government
supervision. The FDA issued a black box
warning about risk to children (which appears on the package). It had little
effect upon sales of
Tamiflu.
13) This side effect
was barely mentioned by our PhARMA friendly corporate press. A Google search
found an article by Reuters
in 2007. Moreover in the article they
used a statement by Roche denying a causal relation of deaths to Tamiflu. The
article also clearly implied that Tamiflu
is effective treatment of the flu.
Under the mountain of positive press the public believes that Tamiflu is
effective. Tamiflu and Relenza (the
knock off drug) are aggressively marketed and their sales continue to grow because
doctors and the public hear of the drugs perceived benefits; not the
ineffectiveness, the need to take the drug within 24 hours of symptoms, and its
side effects. Roche, their thought
leaders, treatment protocols, corporate media, and political connections
have won the market battle. Political connections
explain why governments continue to be stockpile a drug that is 99% ineffective
against the flu (WHO Study above).
14) PhARMA should not
generate & control the information about their drugs—a conflict of interest.
For example, side effects are always
underestimated and only partially known.
The information supplied by thought
leaders in continuing education classes & by sales reps is flawed. Very,
very few doctors are inclined to study
journals and come to an informed opinion as to the costs-benefits of the drugs they
prescribe. Most journal articles are only
available for free
on line at a medical college library. Bias and partial
information makes critical
evaluation often impossible. Only for a
few drugs are there sufficient studies to arrive at a valid conclusion; and to
do so require sifting through hundreds of PhARMA-funded articles and a few by
critics. It takes hundred of hours to
arrive out a reasonable conclusion. Given
these issues doctors who practice medicine in a corporate setting conform to
the PhARMA set norms.
15). Considerable
pressure is placed upon physicians to conform.
In hospitals, nursing homes, and clinics,
corporate administrators set up treatment
protocols. These administrators
receive kick backs from drug-company sales reps for including their drugs in
the protocol. Continued employment is
contingent upon following the protocols.
And thanks to direct-to-consumer advertising, most patients expect to be
heavily medicated with the latest drugs.
Keeping patients is in part based upon fulfilling their
expectations. And if they don’t follow protocol,
and the condition proves resistant to treatment; this could lead to a
mal-practice suit. Given the PhARMA-published
studies justifying the normal course of treatment, it would be difficult for
the doctor to convince a court that the suit lacks merit. Also, mavericks are
less likely to get
referrals from colleagues; or perks from PhARMA. . The
web protocols weave extends to respected professional
societies: “The guidelines represent
the standard of care against which they will be judged in any claims of
malpractice”. The article is on
alteplace, and ER drug for strokes which causes a higher death in 10 out of 12
trials. Without knowing what is best,
following the advice of a thought leaders and treatment protocols is the
doctor’s prudent choice.
16) The power of the
gorilla entails “ostrich behavior” from the FDA, journal editors, and medical
schools as to side effects, etc. This ignorance
as to drug side effect and effectiveness could cheaply be reversed for there are
mountains of long-term data on drugs and side effects, including those not
uncovered in the short-term phase III studies.
They reside in the data banks of the HMOs, Kaiser Permanente, Veterans
Affairs hospitals, corporate hospitals, Medicare, and nursing homes. The long-treatment
records are not available
for inexpensive, scientific analysis. Costly,
long-term phase IV studies don’t fill that gap because PhARMA studies conform
to the standards of tobacco science, and FDA sponsored studies are PhARMA
friendly. Moreover their major studies
have market objectives including finding new uses, expanding uses, quelling concerns
over side effects, showing their drug is safe and effective, and better than
older off-patent one.
The gorilla is in favor of tobacco
sciences and against the use of data banks in an unbiased way.
17) Often journal reports of and the media brouhaha
over side effects muddle the issues. Tamiflu’s statistical association with
a few children
deaths is a typical example. The issue
for a treatment is the harm done compared to the benefits. This requires complete
and accurate data. As stated on page one, the highest standard
consists of an active search for side effects and benefits. This ought to be
done by independent, well-funded scientists with a mandate like that of NICE
supported by a global organization such as the WHO (World Health
Organization). Raw data must be
published. The research performance
should be reviewed by not-for profit, universities which would be involved in
choosing areas to research with the goal of benefiting the public. Many healthful
leads and issues that ought to
be investigated aren’t because the finical incentives for a corporation to
investigate are lacking. We need health
science.
18) How much harm? The theme piped in through direct to consumer
advertising: better living through
miracle drugs brought to you by big PhARMA, who is supervised by the vigilant
FDA that makes PhARMA rattle off a list of side effects at the end of their
television commercial. This is ad doesn’t promote informed choice. How
many Americans remember the 2005 media
coverage of the greatest known drug disaster, Vioxx? In the 6 years on the market
over 100 million
prescriptions and 20 million Americans
took Vioxx. A 2005 estimates place it at
125,000 heart attacks including 55.000 deaths caused by taking long-term the selective
COX-2 inhibitor Vioxx.
The preliminary testing for a patent,
which is short-term, couldn’t uncover the acceleration of atheriogenesis (hardening
of the arties) caused by Vioxx. The phase I & II study of Vioxx which
included 5,000
patients ”the data did not find an increased risk of heart attack or stroke” [it was
short-term, younger population]. To rely
on PhARMA & the FDA to expose side effects is folly.
19) The pattern of folly is
revealed in the unintended results for clinical trials and the responses by Merck
and the FDA. Three major studies were funded
by Merck for to find new uses & promote Vioxx as superior to the other
NSAIDs (Non- Steroidal Anti-Inflammatory Drug). Older NSAIDs inhibit
the COX-1 and COX-2 hormones. A new family, selective NSAIDs, inhibit
only
COX-2. Given this difference, Vioxx and
its 8 knock-offs were marketed as being a more effective analgesic and causing
less adverse gastro-intestinal (GI) events (“these claims have never been convincingly demonstrated”,
Goodman & Gilman 11th Ed.).
The 2000 VIGOR
(Vioxx GI Outcome Research) study sponsored
by Merck compare the efficacy of Vioxx to Naproxen for GI outcomes. “Months
after the preliminary version of VIGOR was published in the New England Journal of Medicine, [NEJM] the journal editors learned that
certain data reported to the FDA were not included in the NEJM article. NEJM
responded with indignation in their
editorial over the deceptive article they published 2 years earlier. But they
knew that withholding information on
side effects is the norm because
the raw data from the study is never in its entirety submitted. NEJM protest
is thus deceptive. The missing
data showed “a significant 4-fold
increased risk of acute myocardial
infarction in rofecoxib
[Vioxx] patients when compared with naproxen patients.”
Merck incongruously argued in journals and to the FDA that Vioxx’s didn’t
cause the excess deaths from heart attacks, but rather that Naproxen prevent
them through its superior thrombolytic function. But the best drugs at
preventing blood clots
only reduce heart attacks (MI) about 30%--not 4-fold. Moreover Naproxen had
never been shown to
provide this type of cardiovascular protection.
In the 2001 APPROVe study
(Adenomatous Polyp PRevention On Vioxx),
a three year trial to see if Vioxx would reduce colon polyps: “A total of 25 [elderly]
patients receiving placebo and 45 receiving Vioxx demonstrated thromboembolic
events…. Including heart attack and stroke appeared statistically evident at 18
months of chronic dosing.” Two years later
these fatal side effects were made public by Merck. A third study 2000 to 01
to see if Vioxx
prevented Alzheimer’s (also using a high risk population) had similar results,
and was terminated early. Merck this
time simply sat on the data for 3 years, and then when it decided to withdraw
Vioxx from the market, Merck then published the fatal results (2004 and
2005). The 2005 article revealed
39 deaths in the Vioxx group and 15 in the placebo group. “These mortality
analyses were neither provided
to the FDA nor made public in a timely fashion”
JAMA.
Troubling is that the Vioxx disaster was exposed in 2000, the FDA
responded with a black-box warning in 2002,
but it was Merck who in Sept. 2004 after sitting on the results of 2 major
trials for 3 years, who voluntarily
withdrew its mega-blockbuster, Vioxx. (With
Tamiflu 8 out of 10 studies were not published.) This pattern is not new. For example the same occurred with DES,
approved by the FDA for birth control in 1942, and subsequent used to prevent
in pregnant women spontaneous abortion—though the evidence for this use was far
from convincing. In 1971 it was found to
increase vaginal and cervical cancers 40 fold in daughter of mothers given
DES. Soon it was shown to cause several
types of internal genital abnormalities in exposed sons and daughters. Nevertheless
it remained available until the in 1997 when Eli Lilly stopped marketing DES. Merck voluntarily withdrew Vioxx in
2004. We need enforced regulations requiring
the collecting of stats on all side effects and their prompt, unbiased
reporting--and major penalties. And we
need an industry unfriendly regulatory agency that will remove promptly such
horrors.
20) Unfortunately the
events surrounding Vioxx as to FDA and Merck’s handling side effects and
efficacy is the norm. It illustrates
inadequacies: the FDA’s protection of the public, the accuracy of journal articles, the
legal system, and how election-funding affects our legislation. It illustrates
how the worse known drug
disaster is quickly forgotten, and its consequence barely punished. We all know
of 9/11, but most have forgotten that
over 20 times as many American died from taking Vioxx. Civil suits were settled
for under $1
billion, a fraction of profits. There were an
estimated 160,000 excess heart
attacks & 55.000 deaths from 1999 to 2003 among the 20 million users, and
more to this day since Vioxx accelerated hardening of the arteries. It also
illustrates the inadequacies of the
system for monitoring long-term side effects (more on that later). Shockingly,
Vioxx’s knockoff Celebrex is still
heavily advertised, though the FDA’s advisory committee unanimously recommended
the prohibition of direct-to-consumer advertising because the evidence showed
the same atherogenesis process. There is no pressing need for the family
of
selective COX-2 inhibitors, which they belong to. These drugs have never soundly been shown to afford more pain relief, arthritic protection,
or cause less hemorrhaging than the non-selective NSAIDs, though PhARMA of
course has produced studies supporting these claims, and the FDA uses these
studies to justify the continued approval of Celebrex and 6 others
21) Illustrative of
the effective control of the production of information concerning drugs and
their usage and the relentless pursuit of profit maximization is the use of
NSAIDs such as Celebrex, naproxen, and ibuprofen. In the last 2 decades it has
come to light
that all of the NSAIDs but aspirin—both
selective and non-selective COX inhibitors--increase the rate of atherogenesis,
and thereby contributes to cardiovascular
disease (CVD). A rough estimate would be for each day on
them is equivalent to smoking a pack of cigarettes—there are major difference
in rate depending on the NSAID. This
analogy to cigarettes highlights their delayed effect on younger users, its
accumulative long-term effect, and the much higher risk for those already with
hardening of the arteries. This is why
only an elderly population could expose this side effect within 2 years. Vioxx
and Celebrex were tested for conditions
requiring an elderly population such as colon polyps and Alzheimer’s disease.
Testing in the elderly should be required for
all drugs. This acceleration of CVD
caused by NSAIDs has resulted in the
American Heart association issuing
a clear warning--see also journal sources. For
the reasons developed (see #1), this fact of CDV risk has minimal market impact,
for PhARMA only sells the fluff. Moreover, aspirin, the only NSAID not to
promote CVD is not recommended for
pain, for inflammation, or to prevent blood clots, because of a “perceived” greater risk of GI events. (And aspirin prevents and increases survival
of most common cancers). Thank you
PhARMA and the FDA.
22) How serious is the
problem
(drug interactions, overdoses, side effects)?
“Various studies have been performed about medical errors…. The
Institute of Medicine (IOM) reports on two
studies estimating the hospital deaths due to medical errors at 44,000 to
98,000 annually, which would place medical errors in the top ten causes of
death in the USA. Barbara Starfield's article in JAMA places the estimates even
higher, citing a total of 225,000 deaths due to iatrogenic causes, which would
place health-care deaths as the 3rd leading cause of death in the USA. Holland
et al (1997) estimates as many as 1 million patients are injured while in the
hospital and approximately 180,000 die as a result, with the majority due to
medication adverse reactions” Doctor Philippe
Even, director of
the prestigious Necker
Institute estimates for France 20,000 death and 100,000 hospital admission--multiple
those
numbers by five to adjust for the US population. The number of early death is
much higher (see
#5 above) because most adverse events are not recognized, and there is no
requirement for physician reporting. This
dismal assessment is supported by the 1871 British census: “males who
lived to adulthood averaged 75 years. Present, male life expectancy in the
U.K. is 77 years for males” (source Wikipedia
“longevity”). With all the life extending interventions: cancer, heart attacks,
diabetes, kidney
stones, trauma, vaccinations, antibiotics, plus healthier working conditions,
sanitation, less smoke from fires, something
must be at work to undo this
progress. Two reasons are tobacco and
obesity which shorten life an average of 7 and 5 years respectively. They afflict
25% and 33% of the population thus
causing an average deduction of 3 years. After that the life-long chemical bath
(called
drugs) shortens on an average lives
by 3 years. Next on the grim list are
the corporate farms and food additives—deduct 2 years. These wipe out
male-adult health care gains since
the 1871 census.
23) In 1970 the
medical
bill accounted for 5 % of GDP, today it is 17% of GDP. The main improvement
in survival since 1970
has been through the injection of clot busting drug in the emergency room of
hospitals for those with heart attack or stroke, and the reduction in tobacco
usage.
PhARMA in our corporatist state has the
highest return on investment of any sector of the economy.
There is a fundamental conflict of
interest the public health and maximizing profits. Though fixes are not
on the political
horizon, since corporations are the shadow government; a public awareness of
the greed & harm done by corporate medicine and the need for a populist
government to fix this mess, this is the first step. Though PhARMA paints
a rosy picture,
we get very little for the major bump in the cost of health care.
24) ADVICE on avoiding side effects: Given
the state of corporate medicine, to
base an evaluation of a drug on the journal literature is a mine field. In journals
positive results, biomarkers,
metastudies, treatment comparisons are all subjected to corporate ethics. To
rely upon a person trained by PhARMA, your physician, is to greatly increase
the risk of making the poor choices. Sincerity
is a poor guide. On the positive side,
learn about your condition from medical textbooks such as Conn’s Current
Therapy, Merck Manual on line, and Wikipedia.
Let your doctor know that you are
not a pill popper. Watch out for
downers (tranquilizers) which have many uses. Don’t ask for drugs for psychological
problems, they are all downers. Mental confusion creates reliance upon your
doctor. Follow worstpills.org
recommend: ask for an older, off-patent
alternative drug, because the side effects are more likely to be revealed. Many
of the older drugs have been proven to
be more effective, though your doctor has been “taught” otherwise. Rely
upon the advice at http://healthfully.org/rc/. There you
will find how and what PhARMA has turned upside down. More are published every
month. Two examples: the truth
about the effect of hormone
replacement therapy for men and women and the protection from cancer through
daily usage of aspirin which activates the bodies system for the destruction of
abnormal cells. These and other topics are
carefully researched in ways that removes the corporate labyrinth of deceit and
arrive at the best-reasoned conclusions
based upon the most reliable evidence. These articles are not faith pitches (trust me I
am an expert), but rather evidenced based, with links to journal articles
(often older ones). As Dr. Philippe Even, the author
of "The Guide to the 4,000 Useful,
Useless or Dangerous Medicines" told The
Guardian: “The
pharmaceutical industry is the most lucrative, the most cynical and the least
ethical of all the industries. It is like an octopus with tentacles that has
infiltrated all the decision-making bodies:
world health organizations, government agencies, parliaments, high
administrations in health and hospitals and the medical profession." My 10
years of developing the website http://healthfully.org has
confirmed that summation. You should
take better care of your health than
your car. And remember the warning by Dr.
Ben Goldacre in Bad PhARMA, page xv:
“Doctors spend forty years practicing medicine with very little formal
education after their initial training.
Medicine changes completely in four decades, and as they try to keep up,
doctors are bombarded with information:
from ads that misrepresent the benefits and risk of new medicines; from
sales reps who spy on patients’ confidential prescribing records; from
colleagues who are quietly paid by drug companies; from ‘teaching’ that is
sponsored by industry; from independent ‘academic’ journals articles that are
quietly written by drug company employees; and worse”
Worse includes treatment protocols that are generated by friends of
PhARMA. Below is an article from the British Medical Journal, June 2013 that
provides insight into the power of PhARMA and its close ties with professional
organizations, in particular the Congress of Neurological Surgeons and the US
National Institute of Health (NHI).
“Interpretation: These data indicate that patients with coronary
heart disease and metabolic syndrome derive incremental benefit from high-dose
atorvastatin [Lipitor] therapy, irrespective of the presence of diabetes.” Given
that sufficient information on the
study was in their hands (BMJ quotation above), the Lancet has
hit its low point. The BMJ article (2006) also pointed the failure to reveal side
effects which has been repeatedly shown to increase with dosage:“However, overall
mortality was not reduced because the smaller number of
cardiovascular deaths in the 80 mg atorvastatin.”
Moreover, the
TNT study failed
to list all causes of
death in the Lancet article.
Their major issues uncovered: “When the team compared published data with the more complete
unpublished trial records, they found apparent inaccuracies in the published
record of the trials. For example, while unpublished trial reports mentioned serious adverse
events (some even
classified as possibly related to oseltamivir), one of the two most cited
publications makes no mention of such effects, and the other states, “there
were no drug-related serious adverse events…. Having pieced together
information from more than 16,000 pages of clinical
trial data and documents used in the
process of licensing oseltamivir, the Cochrane team
raises critical questions about how well the drug works, as well as about its
reported safety profile. While the drug did reduce the time to first alleviation of
symptoms by an average of 21 hours, it did not reduce the number of people who
went on to need hospital treatment.” Given that
bias is norm in journal articles (32% positive bias, see # 6 above), the
evidence for Tamiflu being more effective than a placebo for approval by the
FDA & European regulatory agencies must have been thinner than the journal
article which found a reduction of 21 hours for symptoms of the flu. Why was
this drug stockpiled for avian flu?,
Twenty –one hours won’t have much effect upon the death rate considering that
WHO testing found that 99% of common virus strains are resistant.
“There
were 29 observational studies uncovering Vioxx use is associated with a significant increase in the relative
risk of myocardial infarction…. Why
clinical investigators studying Vioxx did not do more to raise concern is a
fair question that needs to be answered…. Horton never mentions the culpability
of the
medical journals. Concerns over the
toxicity of rofecoxib were first articulated in 2000.4 However, a review of the 1032 publications on
rofecoxib cited in PubMed reveals that, before Merck withdrew the drug, only a
handful of articles raised concerns about its efficacy.”
The older remedies by going
off patent do not become less effective—though PhARMA wants us to believe
so. The published science behind the
older remedies is often superior, for in the 1970’s and earlier. PhARMA
generally didn’t back then own the
results of trials or establish its parameters.
The majority of research was back then done through medical colleges,
and PhARMA merely funded the
studies. Thank you neoliberals (globalizers)--starting with the Reagan
administration--for fixing the system.
|
 |
|
Side effects outline
1.
Introduction,
problem profit maximization versus public safety
2. Safe and
effective, example of 22 elite athletes on statins, not tolerated standard,
expanded & new uses.
3.
Major medical
event, Warfarin counting only 2 or more pints of blood.
4. Self-reporting low
standard, Ideal is an active search for side effects by independent
scientists.
5. Roche sitting on
physicians reports of side effects rather than assessing them and forwarding
them to the EMA and EMA to the FDA’s AERS system. Reports are voluntary
for doctors, and thus
under reported.
6. Phase III studies,
better than a placebo, raw data submitted to FDA, but FDA does not review
journals articles based on raw data Phase iii.
Gap of 32% between raw data in Phase III studies and bias in journal
articles.
7.
Phase IV designed
by marketing for promotion of sales (extend patents, prove safety, new uses,
higher doses, superiority, seeding use, below standards of phase III. Sales
reps and thought leaders use the
results.
EXAMPLES hiding side effects:
8.
Selling benefits,
Lipitor world biggest block buster, stats on usage of statins
9. TNT study statins expands the
category for treatment,
exclusion of those in study, of those once treatment started. Example of unreliable
studies and journal
articles.
10. Meta-study, a
stew of marketing science journal articles.
11. Tamiflu
blockbuster flu treatment, unpublished clinical trials, flu virus resistant to
Tamiflu, lack of positive effects, side effects, not delivering raw data as
agreed to Cochrane.
12. Abnormal behavior
in children and 39 deaths in Japan after Tamiflu, and black-box-warning.
13. Silence in press
over death in children, and positive spin by Router’s article.
14. PhARMA’s control
of information and arriving at reasonable conclusion on the few sufficiently
research topics. Doctors in corporate
setting conform to PhARMA set norms.
15. Pressures upon
doctors to conform to treatment protocols.
16. Ostrich behavior,
tobacco since and the failure to use available data banks of hospitals, nursing
homes, Medicare etc. to reveal side
effects and effectiveness.
17. Brouhaha over
rare serious side effects creates an illusion of FDA protecting public and
media not being bias. Need for a body
like NICE to evaluate treatments.
18. How much harm is
done? Vioxx example.
19. Vioxx
studies and asleep at the wheel; DES asleep at the wheel.
20. Lessons
learnt from Vioxx disaster.
21. No
need for COX-2 inhibitors, NSAIDs causing CVD, superiority of aspirin.
22). How
serious is the problems? Drug
error deaths, side effects deaths, British census of 1871, and 2 year gain in
life for mature males compared to now,
reasons, obesity, tobacco, drugs, corporate farms and food additives.
23. Improvement
in survival since 1970 has been based on injection of clot busting drug for MIs
and strokes, and reduction in cigarette smoking.
24. Advice,
don’t be a pill popper, many older off-patent drugs are safer and more
effective, go towww.worstpills.org and http://healthfully.org/rc/index.htm,
and live healthfully. Be a
skeptic and remember “the pharmaceutical industry is the most lucrative, the most
cynical and the least ethical of all the industries. It is like an octopus with
tentacles that has infiltrated all the decision-making bodies: world
health organizations, government
agencies, parliaments, high administrations in health and hospitals and the
medical profession."
^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^
|
|
|
|
|
|
|

|
^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^
There is an ever growing
revulsion by physicians over the corporate takeover. Four weeks before
this, BMJ publish an
article by a physician who was invited by a collogue to be part of an advisory
panel. He describes how at the beginning
of the meeting, without discussion of the matter before them, the panel voted
to approve the new protocol. A panel of
PhARMA friendly physician will not review a protocol in the public’s
interest. Below is one more example of
how business is done. This article
points out that the fear of malpractice suit compels physicians to follow
protocols--jk.
FEATURE
Evidence Based Medicine
Why we
can’t trust clinical guidelines
BMJ 2013; 346 doi: http://dx.doi.org/10.1136/bmj.f3830 (Published
14 June 2013). Cite this as: BMJ 2013;346:f3830
Jeanne Lenzer,
medical investigative journalist
jeanne.lenzer@gmail.com
Despite repeated calls to prohibit or limit conflicts of
interests among authors and sponsors of clinical guidelines, the problem
persists. Jeanne Lenzer investigates
On 13
April 1990, in an unprecedented action, the US National Institutes of Health faxed
a letter to every physician in the US
on how to correctly prescribe a breakthrough treatment for acute spinal cord
injury. Many neurosurgeons were sceptical of the evidence that lay behind
the new recommendation to give high dose steroids, yet when two respected organisations
released a review and a guideline
recommending the treatment, they felt obliged to give it. Now, over two decades
later, new
guidelines warn against the serious harms of high dose steroids. This case and others
like it point to the ethical
difficulties that doctors face when biased guidelines are promoted and raise
the question: why do processes intended to prevent or reduce bias fail?
Doctors who are sceptical about the
scientific basis of clinical guidelines have two choices: they can follow
guidelines even though they suspect doing so will cause harm, or they can
ignore them and do what they believe is right for their patients, thereby risking
professional censure and
possibly jeopardising their careers.1 2 34 This is no mere theoretical dilemma;
there is evidence that even when doctors believe a guideline is likely to be
harmful and compromised by bias, a substantial number follow it.5
Disturbing precedent
In the early 1990s, high dose steroids
became the standard of care for acute spinal cord injury,6 reinforced by a Cochrane review. The
Cochrane Collaboration, is widely known to have strict standards concerning
conflicts of interest, yet in this case the collaboration permitted Michael
Bracken, who declared he was an occasional consultant to steroid manufacturers
Pharmacia and Upjohn, to serve as the sole reviewer.7 He was also the lead researcher on the
single landmark study, published in the New England Journal of Medicine,8 used to support the Cochrane review.
Neurosurgeons were not convinced. Many expressed concern about high rates of
infection, prolonged hospital stays, and death with high dose steroids.9 10 One expert estimated that more
patients had been killed by the treatment in the past decade than died in the
9/11 World Trade Center attacks.5
A poll of over 1000 neurosurgeons showed
that only 11% believed the treatment was safe and effective. Only 6% thought it should
be a standard of
care. Yet when asked if they would continue prescribing the treatment, 60% said
that they would. Many cited a fear of malpractice if they
failed to follow “a standard of care.”5
That standard was reversed this March, when
the Congress of Neurological Surgeons issued new guidelines. The congress found
that, “There is no Class I or Class II medicine evidence supporting the benefit
of [steroids] in the treatment of acute [spinal cord injury]. However, Class I, II,
and III evidence
exists that high-dose steroids are associated with harmful side effects
including death.”11
Manufacturing consensus
Guidelines
are usually issued by large panels of authors representing specialty and other
professional organisations. While it might seem difficult to bias a guideline
with so many experts participating under the sponsorship of large professional
bodies, a worrying number of cases
suggests that it may be common.
A
recent survey found that 71% of chairs of clinical policy committees and 90.5%
of co-chairs had financial conflicts.12 Such conflicts can have a strong
impact: FDA advisers reviewing the safety record of the progestogen
drospirenone voted that the drug’s benefits outweighed any risks. However, a
substantial number of the advisers had ties to the manufacturer and if their
votes had been excluded the decision would have been reversed.13 [Used in
birth control pills, Drospirenone causes a 6 fold increase in blood clot—the most
common serious side effect of birth control pills.]
Biased guidelines can have powerful and
wide ranging effects. Thousands of guidelines have been issued,14 and, when promulgated by highly
respected professional societies, they sometimes serve as de facto “standards
of care” that may be used to devise institutional protocols, to develop
measures of physician performance, and for insurance coverage decisions.
Guidelines may influence the medicines selected for inclusion on drug formularies
and may be used as a “reliable authority” to support expert testimony in
malpractice suits.4 Eighty
four per cent of doctors say they are concerned about industry influence over
clinical guidelines,12 yet
the fear of malpractice suits puts many in an untenable position of following
guidelines they believe are flawed or dangerous to patients.
Despite repeated calls to prohibit or limit
conflicts of interests among guideline authors15 16 and their sponsors, most guideline
panellists have conflicts,17making the
guidelines they issue less than reliable.
Exuberant claims for alteplase
in stroke
A similar scenario may be playing out for
the use of the thrombolytic drug alteplase in acute stroke. Earlier this year,
the American College of Emergency Physicians with the American Academy of
Neurology (jointly)18 and the American Heart Association,19 separately, issued grade A level of
evidence guidelines for alteplase in acute stroke. The simultaneous
recommendation by three respected professional societies would seem to indicate
overwhelming support for the treatment and consistent evidence. However, an
online poll of 548 emergency physicians showed that only 16% support the new
guidelines.20 Although the poll was not scientific,
other surveys show substantial scepticism among emergency physicians and the
treatment remains contentious.21 22 23 24 25 26 27
Guideline authors say that opposition to
the guidelines is insubstantial. Andy Jagoda, a member of the guideline
committee and professor and chair of emergency medicine at Mount Sinai School
of Medicine, said that “almost all” resident physicians “believe in tPA
[alteplase] for stroke.” Another guideline author, Steven R Messe, assistant
professor of neurology at the Hospital of the University of Pennsylvania and
the Pennsylvania Hospital, told the BMJ that
“only a small, vocal minority [of emergency physicians] are opposed.”
An earlier survey claimed that emergency
physicians don’t oppose alteplase for stroke. At a glance, the claim seemed
justified: the poll found that 83% of the doctors surveyed said they would give
the treatment.21 However, when asked whether “the
science supports the use of tPA [alteplase],” only 49% agreed.
Alteplase was approved for acute stroke
after the 1995 National Institutes of Neurologic Diseases and Stroke (NINDS)
trial showed a 13% absolute reduction in disability.28 Advocates quickly began to promote the
treatment with exuberant claims. The American Heart Association said it could
“save lives,” a claim the organisation was forced to withdraw in 2002 when it
was pointed out that no study had shown a mortality benefit.29 In 2007, leading stroke experts with
industry ties repeated the “saves lives” claim in the New
York Times, suggesting that far too few stroke patients were
receiving the drug, largely because of resistance among emergency physicians.
The newspaper later published a brief correction stating there was no evidence
to support the claim that the drug saved lives.30
But as
with steroids for acute spinal cord injury, claims of benefit rest on science
that is contested. Sceptics say that
baseline imbalances, the use of subset analyses, and chance alone could account
for the claimed benefit.24 26 31 32 33 They
also note that only two of 12 randomised controlled trials of thrombolytics
have shown benefit and five had to be terminated early because of lack of
benefit, higher mortality, and significant increases in brain haemorrhage.33
In addition, the guideline committee did
not include the largest study of the treatment to date in its analysis. Messe,
who was one of the guideline’s authors and a spokesperson until April 2011 for
Boehringer Ingelheim, Genentech’s European marketing partner, told the BMJ that the joint panel did not include
the International Stroke Treatment-3 (IST-3) Trial because the outcome “showed
a benefit” among subgroups and because the patients treated were not the same
population as in the NINDS trial. However, the effect on the primary outcome in
IST-3 (treatment of stroke from 0-6 hours) was actually negative, and the
claimed benefits were based on secondary, exploratory analyses. When this was
pointed out, Messe acknowledged that the primary outcome was negative and said,
“No one has claimed, nor do we recommend, treatment up to 6 hours.”
The new grade A recommendation by the
American College of Emergency Physicians is seen as particularly surprising
because emergency physicians have been the strongest critics of the treatment.
In a survey of 1105 emergency doctors, 40% said they were “not likely to use”
alteplase for acute stroke even under the ideal conditions recommended by the
NINDS protocol.34 Two thirds of those doctors cited the
risk of symptomatic intracerebral haemorrhage as the factor that most concerned
them. A quarter cited the lack of clear treatment benefit.34 Their concerns seem understandable in
light of a Cochrane review of pooled effects that showed alteplase increased fatal
intracerebral haemorrhage nearly fourfold,
and that thrombolytics overall were associated with a significant increase in
mortality by the end of follow-up, representing an extra 30 deaths per 1000
treated patients.35
Curt Furberg, a prominent methodologist and
former Food and Drug Administration adviser, told the BMJ:
“The most powerful evidence comes from the Cochrane pooled analysis.” Furberg
objected to the use of subgroup analyses to prove benefit, saying, “When
clinical trial results are heterogeneous, it’s important to look at the
totality of evidence. You should never draw firm conclusions from post hoc
analyses. You can’t just select data that supports the thesis you like by
asking, ‘How do the results look at 2 hours? How about 2 hours and 10 minutes?
How about 3 hours?’ By chance alone you will find something that supports your
bias.”
Best guidelines influence can
buy: how it happens
Proponents
of alteplase have launched
projects to ensure uptake of the guidelines in the US, such as the development
of “stroke certified hospitals,” which require hospitals to commit resources to
enable rapid administration of alteplase to eligible stroke patients. Since ambulances
divert
patients with suggestive symptoms to stroke certified hospitals, the project
has substantial financial ramifications. These efforts, and others like the “Brain
Attack” campaign, have been
actively supported by the American Heart Association and American Stroke
Association, which “partnered” with the Joint Commission (a quasi-governmental
agency that accredits hospitals) to promote hospital stroke certification. Genentech,
Boehringer Ingelheim and Novo
Nordisk, which market alteplase, have contributed tens of millions of dollars
to the associations.
In its newly released guidelines, the
American Heart Association states that it “makes every effort to avoid any
actual or potential conflicts of interest that may arise as a result of . . . a
business interest of a member of the writing panel.” However, according to
their conflict of interest disclosure statements, 13 of the 15 authors had ties
to the manufacturers of products to diagnose and treat acute stroke; 11 had ties
to companies that market
alteplase.19 In 2010, two years after the
association launched this guideline panel, it revised its financial conflicts
policy; in the future, neither committee chairs nor the majority of its
guideline writing members may have any relevant ties to industry.
Concern about the credibility of guidelines
led the Institute of Medicine to recommend that ideally no guideline authors
should have financial conflicts of interest.14 If individuals who have professional
conflicts that can’t be divested (for example, specialists whose career depends
on treating a certain condition) are included, the institute recommends that
they “should represent not more than a minority” of the panellists.14 [Such requirements by the professional organizations
is mere window dressing, for business continues as before, there hasn’t been a flock
of changes in protocols.]
In the guidelines issued jointly by the
American College of Emergency Physicians and the American Academy of Neurology,
three of eight panellists disclosed ties to the manufacturers. However, seven
had either direct ties to the manufacturer or indirect ties, knowingly or not,
through affiliations with the Foundation for Education and Research in Neurological
Emergencies (FERNE), which provides unrestricted continuing medical education
grants (table⇓). Guideline readers were unlikely to know that
according to its 2008 tax return, 100%
of the $97 000 donated to the foundation that year came from drug companies,
including $50 000 from Genentech. The foundation president and founder, Edward
P Sloan, is an outspoken advocate of alteplase for stroke.36
Competing interests of
authors of American College of Emergency Physicians and the American Academy of
Neurology guidelines on alteplase
|
Author
|
Competing interest
|
Disclosed
|
|
Edlow
|
FERNE
|
Yes
|
|
Smith
|
Genentech
|
Yes
|
|
Stead
|
No
|
|
|
Gronseth
|
Boehringer Ingelheim
|
Yes
|
|
Messe
|
Boehringer Ingelheim
|
Yes
|
|
Jagoda
|
FERNE only
|
|
|
Wears
|
Speaker for FERNE* (not stroke
related)
|
No
|
|
Decker
|
Adviser and speaker for FERNE*
|
No
|
For all guidelines, the overwhelming majority of committee chairs and cochairs have ties to
industry,12 and selection of panellists with
desired viewpoints can make a wished for outcome a foregone conclusion.
Committee stacking may be one of the most powerful and important tools to
achieve a desired outcome. Seven of the
eight panellists had previously published or lectured on the merits of
alteplase for stroke. The eighth panellist, Robert Wears, described himself
as an “agnostic” but added that he was “surprised” that he was named as an
author since he had resigned from the committee six years earlier. Not one
sceptic was included on the panel. In response to a question
about
whether any known sceptics were invited to be on the committee, a spokesperson
for the American Academy of Neurology said, “A potential panel member’s opinion
on a topic does not determine eligibility for participation on an American
Academy of Neurology guideline author panel. The guideline development process
is evidence based.”
Wears, a highly respected methodologist and
professor of emergency medicine at the University of Florida Health Sciences
Center, had been the methodologist for the committee. He told the BMJ that
he
resigned in part because he was growing increasingly “disillusioned” with the
guideline process. When asked why Wears’ name appeared as one of the committee
members, Rhonda Whitson, clinical practice manager for the college told the BMJ, “He
may
have thought his role on the tPA panel ended sooner than it did . . . However,
he did participate throughout the project as needed for his role.”
A spokesperson for the Annals
of Emergency Medicine, which published the clinical policy,
explained how Wears’ name was able to appear in the journal. She told theBMJ that it
does not peer review the college’s clinical policies; nor does it vet the
authors or members of the development panel.
Widespread problem
Many other conflicted guidelines have come
to light in recent years. In 2006, the New
England Journal of Medicine published an article warning against
aggressive treatment of anaemia with erythropoietin in patients with kidney
disease. Patients treated aggressively had increased rates of heart failure and
need for dialysis.37Yet
guidelines issued in 2007 by the National Kidney Foundation, which received
multimillion dollar donations from companies that make erythropoietin,
recommended aggressive treatment that would increase the number of patients
receiving the drug.38
In 2004, newly issued cholesterol
guidelines greatly expanded the number of people for whom treatment is
recommended. A firestorm broke out when it was learnt that all but one of the
guideline authors had ties to the manufacturers of cholesterol lowering drugs.39
Yet
these and other guidelines continue to be followed despite concerns about bias,
because as one lecturer told a meeting on geriatric care in the Virgin Islands
earlier this year, “We like to stick within the standard of care, because when
the shit hits the fan we all want to be able to say we were just doing what
everyone else is doing—even if what everyone else is doing isn’t very good.”
Notes
Cite this as: BMJ 2013;346:f3830
Footnotes
·
Competing
interests: I have read and understood the BMJ Group
policy on declaration of interests and have no relevant interests to declare.
·
Provenance
and peer review: Commissioned; not externally peer
reviewed.
References
1.
↵ Cundiff D. The story behind a whistleblower
doctor license reinstatement hearing. KevinMD.com, 6 January 2010. www.kevinmd.com/blog/2010/01/story-whistleblower-doctor-license-reinstatement-hearing.html.
2.
↵ Garber AM. Evidence-based guidelines as a foundation for
performance incentives. Health Aff (Millwood) 2005;24:174-9.
Abstract/FREE Full Text
3.
↵ Timmermans S, Mauck A. The promises and pitfalls of
evidence-based medicine. Health Aff (Millwood)2005;24:18-28.
Abstract/FREE Full Text
4.
↵ Molloy S, Middleton F, Casey AT. Failure to administer
methylprednisolone for acute traumatic spinal cord injury—a prospective audit
of 100 patients from a regional spinal injuries unit. Injury2002;33:575-8. CrossRefMedlineWeb of Science
5.
↵ Lenzer J. NIH secrets: study break. New Republic2006 Oct
6. www.newrepublic.com/article/national-institutes-health-fred-geisler-spinal-cord.
6.
↵ Canadian Association of Emergency Physicians. Steroids in
acute spinal cord injury. CJEM2003;5:7-9.
Medline
7.
↵ Bracken MB. Steroids for acute spinal cord injury. Cochrane
Database Syst Rev2009;1:CD001046.
8.
↵ Bracken M, Shepard MJ, Collins WF, Holford TR, Young W,
Baskin DS, et al. A randomized, controlled trial of methylprednisolone or
naloxone in the treatment of acute spinal-cord injury. N Engl J Med1990;323:1207-9. CrossRefMedline
9.
↵ Nesathurai S. Steroids and spinal cord injury: revisiting
the NASCIS 2 and NASCIS 3 trials. J Trauma1998;45:1088-93.
MedlineWeb of Science
10.
↵ Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green
B, et al. A critical appraisal of the reporting of the National Acute Spinal
Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord
injury. J Spinal
Disord2000;13:185-99.
CrossRefMedlineWeb of Science
11.
↵ Hurlbert RJ, Hadley MN, Walters BC, Aarabi B, Dhall SS, Gelb
DE, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery2013;72:93-105. CrossRefWeb of Science
12.
↵ Kung J. Failure of clinical practice guidelines to meet
institute of medicine standards: two more decades of little, if any, progress. Arch
Intern
Med2012;172:1628-33. CrossRefWeb of Science
13.
↵ Lenzer J, Epstein K. The Yaz men. Washington Monthly2012 Jan
9. www.washingtonmonthly.com/ten-miles-square/2012/01/the_yaz_men_members_of_fda_pan034651.php.
14.
↵ Institute of Medicine. Clinical practice
guidelines we can trust. National Academies Press, 2011.
15.
↵ Ransohoff DF, Pignone M, Sox HC. How to decide whether a clinical
practice guideline is trustworthy. JAMA2013;309:139-40.
CrossRefMedlineWeb of Science
16.
↵ Guyatt G, Akl EA, Hirsh J, Kearon C, Crowther M, Gutterman
D, et al. The vexing problem of guidelines and conflict of interest: a
potential solution. Ann Intern Med2010;152:738-41. Medline
17.
↵
Gale EA.
Conflicts of interest in guideline panel members. BMJ2011;343:d5728. FREE Full Text
18.
↵ Clinical policy: use of intravenous tPA for the management
of acute ischemic stroke in the emergency department. Ann Emerg Med2013;61:225-43. CrossRef
19.
↵
Jauch EC,
Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines
for the early management of patients with acute ischemic stroke: a guideline
for healthcare professionals from the American Heart Association/American
Stroke Association. Stroke2013;44:870-947.
Abstract/FREE Full Text
20.
↵
Poll on
support for new guidelines on tPA for stroke. SurveyMonkey2013. www.surveymonkey.com/s/CBF2ZDB.
21.
↵ Scott PA, Xu Z, Meurer WJ, et al. Attitudes and beliefs of
Michigan emergency physicians toward tissue plasminogen activator use in
stroke: baseline survey results from the Increasing Stroke Treatment through
Interactive behavioral Change Tactic (INSTINCT) Trial hospitals. Stroke2010;41:2026-32.
Abstract/FREE Full Text
22.
↵ Fatovich DM. Believing is seeing: stroke thrombolysis
remains unproven after the third international stroke trial (IST-3). Emerg
Med
Australas2012;24:477-9. CrossRefMedline
23.
↵ Hoffman JR, Cooper RJ. How is more negative evidence being
used to support claims of benefit: the curious case of the third international
stroke trial (IST-3). Emerg Med Australas2012;24:473-6. CrossRefMedline
24.
↵ Hoffman JR, Schriger DL. A graphic reanalysis of the NINDS
Trial. Ann Emerg Med2009;54:329-36.
CrossRefMedlineWeb of Science
25.
↵
Klauer K.
History repeating: questioning tPA. Emerg Physicians Monthly, 2012 Nov. www.epmonthly.com/columns/in-my-opinion/history-repeating-questioning-tpa/.
26.
↵
Newman D. The guideline, the science, and the gap. Scientific medicine and
research translation.17 April 2013. http://smartem.org/content/guideline-science-and-gap.
27.
↵ Newman DH, Shreves AE. Thrombolysis in acute ischaemic
stroke. Lancet2012;380:1053-4; author
reply 1054-5. Medline
28.
↵ National Institute of Neurological Disorders. Stroke rt
PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med1995;333:1581-7. CrossRefMedlineWeb of Science
29.
↵
Lenzer J.
Alteplase for stroke: money and optimistic claims buttress the “brain attack”
campaign. BMJ2002;324:723-9. FREE Full Text
30.
↵ Kolata G. Lost chances for survival, before and after
stroke. New York Times2007 May 28. www.nytimes.com/2007/05/28/health/28stroke.html.
31.
↵
Radecki RP.
Skepticism about thrombolytics in stroke is not unreasonable. Nat Rev
Neurol2011;8:176. Medline
32.
↵ Fatovich DM. Response to the recent best evidence topic on
the use of thrombolysis in stroke. Emerg Med J2012;29:82.
FREE Full Text
33.
↵ Newman D. Thrombolytics for acute ischemic
stroke. TheNNT.com 2012. www.thennt.com/nnt/thrombolytics-for-stroke/.
34.
↵ Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern
LB. Survey of emergency physicians about recombinant tissue plasminogen
activator for acute ischemic stroke. Ann Emerg Med2005;46:56-60.
CrossRefMedlineWeb of Science
35.
↵ Wardlaw JM, Murray V, Berge E, Del Zoppo GJ. Thrombolysis
for acute ischaemic stroke. Cochrane Database Syst Rev2009;4:CD000213.
Medline
36.
↵ Sloane EP. Clinical Use of tPA in acute
ischemic stroke. www.uic.edu/com/ferne/pdf/chile1103/sloantpa_acep_chile1103_lecture.pdf.
37.
↵ Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M,
et al. Correction of anemia with epoetin alfa in chronic kidney disease. N
Engl J Med2006;355:2085-98.
CrossRefMedline
38.
↵ Armstrong D. Medical journal spikes article on industry ties
of kidney group. Wall
Street Journal 2006 Dec 26. http://online.wsj.com/article/SB116709354076459273.html.
39.
↵ Abramson JE, Barnard RJ, Barry HC, Bezruchka
S, Brody H, Brown DL, et al. E.petition to the National Institutes of Health
seeking and independent review panel to re-evaluate the national cholesterol
education project guidelines. 2004. http://cspinet.org/new/pdf/finalnihltr.pdf.
INTERNAL SITE SEARCH ENGINE by Google
Looking for a topic, use Google Internal Search
Engine
^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^ Remember that
pharma is in the business of treating illness. There claim of preventing illness is in most cases mere marketing.
Disclaimer: The
information, facts, and opinions provided here is not a substitute for
professional advice. It only indicates
what JK believes, does, or would do. Always
consult your primary care physician for medical advice, diagnosis, and
treatment.
|
|
|